ACE Report: How to Get Past Temperature from an Ice Core
Open Google Doc of How to Get Past Temperature from an Ice Core
By Rebecca Anderson, ACE Director of Education
In a word: isotopes.
Okay… what is an isotope?
An isotope of an element has a different number of neutrons in its nucleus. Sounds complicated, I know!
Here’s an example: Carbon (C) typically has 6 protons and 6 neutrons, so it’s carbon-12 or 12C. But there’s also carbon-14 or 14C that has 2 extra neutrons. It’s this isotope of carbon that radioactively decays and allows archaeologists to date artifacts and find out how old they are.
Not all isotopes are unstable and radioactively decay, though, like 14C. Some are completely stable and exist out there in the natural world just fine. There’s just a lot less of them than the standard version of that element.
One example of this is oxygen-18. Typically, oxygen has 8 protons and 8 neutrons in its nucleus, making it 16O. But 18O has 2 extra neutrons. About 0.2% of all the oxygen in the world is 18O. Not much!
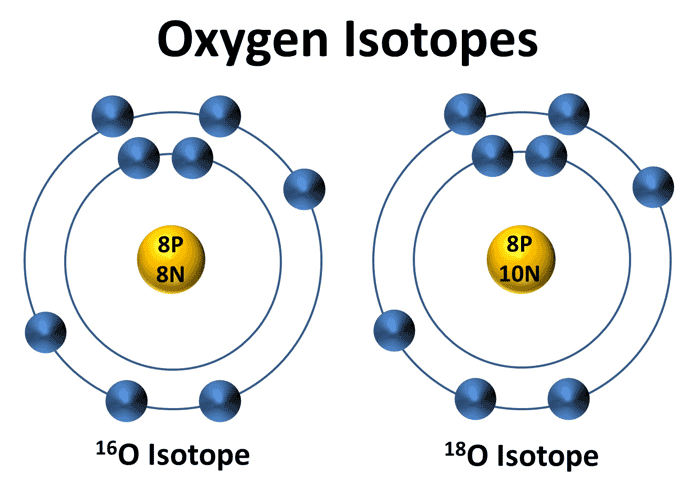
Here’s the key: because 18O has 2 extra neutrons, it’s a very tiny bit heavier than 16O.
And this makes it behave just a bit differently. When that 18O is the O of H2O, for example, being a little bit heavier makes it a little less likely to evaporate than regular H2O and, if it does evaporate, a little more likely to fall out as rain or snow. Being heavier, it just wants to be in that solid or liquid state more than regular H2O.
This process of 18O behaving differently than 16O is called fractionation. And how differently 18O behaves is related to temperature. At colder temperatures, 18O wants to rain or snow out even more.
This relationship to temperature means that scientists can measure the ratio of 18O to 16O in the ice of the ice core and determine what the temperature was at that spot at that time in the past. (And they know when that was in the past because of counting annual variations in the ice and from volcanic eruptions – see back to the science report on “How to date an ice core.”)
Complicated? Yes. The full picture is quite a bit more complicated than what I’ve explained here. If you want to learn more about this, this website from NASA is the best one I’ve found for explaining this in an understandable way (and they go into more detail on the complications). They’ve got some cool diagrams here, too. Definitely worth checking out.
How to Get Past Temperature From an Ice Core
Student Worksheet
Name:
1. What is an isotope?
2. What is Carbon-14 (14C) used for? What’s different between it and 12C?
3. What is Oxygen-18 (18O). How is it different from 16O?
4. How much 18O is found in the environment?
5. What do we call the difference in behavior between 18O and 16O?
6. Which isotope prefers to be in its solid state slightly more, 18O or 16O?
7. How does the 18O:16O ratio help us determine past temperature?
